2020 Volume 45 Issue 10 Pages 611-617
2020 Volume 45 Issue 10 Pages 611-617
Acute paraquat poisoning (APP) is a serious public health problem with a high mortality rate and there is no specific antidote for APP in clinical. Early haemoperfusion (HP) treatment is effective in APP rescue. In this study, we compared the influence of routine HP and continuous HP on the survival rate and the treatment of pulmonary fibrosis in mild and moderate APP patients. Eighty-two cases of mild and moderate APP patients who were admitted to our hospital from January of 2017 to December of 2018 were selected. All patients were randomly divided into a routine haemoperfusion (HP) group (n = 40) and a continuous haemoperfusion (CHP) group (n = 42). Compared with the HP group, the 28-day survival rate of mild and moderate APP patients was elevated in the CHP group. Blood N-terminal procollagen Ш propeptide (PIIINP) levels in APP patients were positively related with paraquat (PQ) concentration (r = 0.309, P = 0.000). There were statistically significant differences in the levels of PIIINP, Collage TypeIV (CIV), transforming growth factor-beta 1 (TGF-β1), malondialdehyde (MDA), superoxide dismutase (SOD) activity and sequential organ failure assessment (SOFA) score between the two groups both on the third and seventh days after treatment, and the treatment effect of the CHP group on pulmonary fibrosis in APP patients was better than that of the HP group. In conclusion, CHP treatment had a significant therapeutic effect on mild and moderate APP patients, which could effectively improve the survival rate and relieve pulmonary fibrosis.
Paraquat (PQ) is commonly used as a herbicide in some Asian countries. Because of its low toxic dose, widespread availability and low cost, PQ has been a widely used suicide agent, and acute PQ poisoning (APP) is a serious public health problem with a mortality rate of 60%-70% and lack of specific antidote in clinical (Gil et al., 2014).
After oral ingestion of PQ, alveolar epithelial cells targeted transport PQ into the lungs, and the accumulation of PQ produces a mass of oxygen free radicals and inflammatory factors, leading to acute lung injury, pulmonary fibrosis, and multiple organ failure (Zhang et al., 2019). Several researchers reported that the lungs are the target organ of PQ and severe pulmonary fibrosis is the major cause of death in the case of APP (Hoet et al., 1994; Shen et al., 2017a).
Since PQ will be distributed to various organs through blood circulation after oral absorption and cause organ failure, early elimination of toxins is crucial for the treatment of APP. Haemoperfusion (HP) is a usual method to remove toxins from the blood circulation in the clinical and the application of HP in the early stage of APP can largely eliminate PQ from the blood. It has been reported that the treatment of HP during the first 4 hr of APP could reduce the concentration of PQ by about 70% (Wang et al., 2017a) and greatly improve the survival rate of patients with APP (Wang et al., 2017b). Though many pieces of evidence showed that HP was necessary for APP rescue, the frequency and duration of HP during APP rescue are still controversial. Here, in this study, we compared the influence of routine HP and continuous HP on the survival rate of APP patients and the treatment of pulmonary fibrosis. Our findings indicated that continuous HP evaluated the survival rate and relieved pulmonary fibrosis in mild and moderate APP patients, proving the application value of continuous HP for the clinical treatment of APP.
Eighty-two patients with mild and moderate APP (44 females and 38 males, with an average age of 36.79 ± 19.63 years) were enrolled in this study between January of 2017 and December of 2018 at the Emergency Department of Harrison International Peace Hospital Affiliated to Hebei Medical University. The inclusion criteria of all patients were based on the “Taishan consensus” on diagnosis and treatment of paraquat poisoning (2014): the concentration of PQ ≤ 30 mg/L, and none of the patients had a prior history of a serious heart or lung disease, infectious disease, sepsis, cerebrovascular disease, or metabolic disease. Also, all the patients were first treated in our hospital, and all of them were PQ poisoned through the mouth. The time between taking the poison and admission was less than 4 hr, and the PQ oral dose was 10-50 mL.
After admission, the PQ concentration was detected and the results can be obtained in 20 min. According to the detection results, a total of 11 patients were excluded due to PQ concentrations higher than 30 mg/L (this segment of patients will die in the early days of admission from multiple organ failure and cannot reflect the therapeutic effect of different treatment methods). Then, all eligible patients were randomly divided into a routine haemoperfusion (HP) group (n = 40) and a continuous haemoperfusion (CHP) group (n = 42). The clinical information of each group is exhibited in Table 1 and there are no statistically significant differences in the general data of each group in gender composition, age, body mass index, degree of poisoning, the dose of poison, PQ concentration in blood, time from poisoning to the first gastric lavage, and time from poisoning to the first irrigation. All procedures of this study were approved by the hospital ethics committee, which met the requirements of ethics and obtained informed consent from the patient’s family.

| HP group | CHP group | |||||
|---|---|---|---|---|---|---|
| Number | 40 | 42 | ||||
| Age (yr) | 36.54 | ± | 19.61 | 37.61 | ± | 18.54 |
| Gender (M/F) | 18/22 | 20/22 | ||||
| Body mass index (kg/m2) | 23.45 | ± | 3.17 | 23.63 | ± | 2.68 |
| Degree of poisoning (mild/moderate) | 19/21 | 21/21 | ||||
| Drug doses (mL) | 26.45 | ± | 5.64 | 26.35 | ± | 6.08 |
| Blood PQ concentration (mg/L) | 15.24 | ± | 7.23 | 14.93 | ± | 7.58 |
| Time from poisoning to HP (hr) | 3.96 | ± | 0.32 | 4.11 | ± | 0.27 |
| Time from poisoning to gastric lavage (hr) | 1.25 | ± | 0.24 | 1.37 | ± | 0.23 |
HP group: routine haemoperfusion group; CHP group: continuous haemoperfusion group.
All enrolled patients underwent gastric lavage, catharsis, cyclophosphamide, large dose of adrenal glucocorticoid, reduced glutathione, vitamin E, vitamin C, fluid supplement, diuretic, and organ function support therapy. Within 4.5 hr of poisoning, on the basis of conventional therapy, APP patients received HP or CHP treatment. In the HP group (n = 40), the patients underwent HP (Jf-800A Blood Perfusion Device, Jianfan Biotechnology Co., Ltd., Zhuhai, China) for 2 hr and this operation was repeated three times at an interval of 8 hr. In the CHP group (n = 42), haemoperfusion was performed for 4 hr firstly. Then the PQ concentration was tested every 2 hr, and this operation was continued until PQ composition could not be detected in the blood. The blood flow rate of the HP and CHP groups was 180 mL/min and resin was used as absorbing material in the HP and CHP groups.
MeasurementsTen milliliters of elbow venous blood was collected from all patients at three time points: pre-treatment, third and seventh days after treatment and then centrifuged at 3000 rpm/min for 10 min to extract the serum. The levels of N-terminal procollagen Ш propeptide (PIIINP) and Collage TypeIV (CIV) were detected by ELISA kit (Beijing Reheat Biotechnology Co., Ltd., Beijing, China). The level of TGF-β1 was detected by the ELISA kit purchased from Shiruike Biological Co., Ltd. (Shanghai, China). The content of MDA was measured by the MDA assay kit (TBA method) and the activity of SOD was measured by the SOD assay kit. The MDA assay kit and the SOD assay kit were all purchased from Nanjing Jincheng Institute of Biological Engineering (Nanjing, China).
Blood PQ concentration was detected by LC-MS gas chromatography at the Poison Detection Center of Hengshui Health and Epidemic Prevention Station. The sequential organ failure assessment (SOFA) score and the survival rate at 28 d were recorded. High-resolution computed tomography (HRCT) of APP patients’ lungs were conducted at pre-treatment, third and seventh days after treatment.
Statistical analysisAll data were statistically analyzed using SPSS17.0 software. All independent samples are normally distributed. Data were expressed as mean ± standard deviation (± s), and repeated-measures analysis of variance or one-way analysis of variance was used. The t-test and the χ2 test were used to compare between groups. The χ2 test was used to compare the rates. The Log Rank test was used to compare the survival rate. The correlation analysis was performed by the Pearson correlation test. Test level α = 0.05, P < 0.05 was considered statistically significant.
As shown in Fig. 1, compared with mild APP patients, the moderate APP patients exhibited a higher blood PQ concentration at admission, showing that the level of blood PQ concentration could reflect the severity of poisoning.
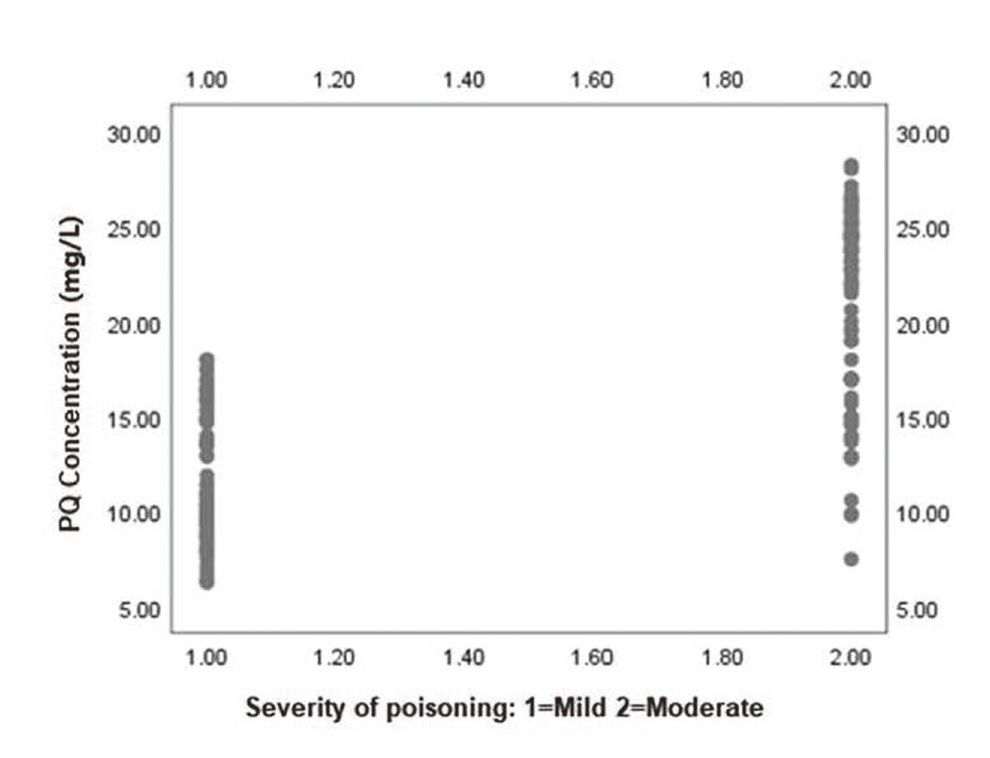
The correlation between blood PQ concentration at the admission of patients and severity of poisoning (n = 82). P = 0.000.
The PQ concentration in the HP group was measured at 3 time points (before treatment, 8 and 24 hr after treatment) and the concentration was 17.58 ± 5.62 mg/L, 9.54 ± 3.47 mg/L, and 4.86 ± 0.98 mg/L, respectively. The PQ concentration in the CHP group was measured at 4 time points (before treatment, 4, 6, 8 hr after treatment) and the concentration was 15.71 ± 6.73 mg/L, 6.35 ± 2.46 mg/L, and 4.02 ± 0.83 mg/L. The PQ concentration in the CHP group could not be detected at 8 hr after treatment. Therefore, compared with HP treatment, CHP treatment could remove the PQ from the blood faster.
Continuous HP improved the survival rate of patients with APPWe calculated the fatality rate of mild and moderate APP patients on the 28th day of admission, finding that the fatality rate of the HP group was 65% (26/40) and the CHP group was 45.24% (19/42), showing a statistically significant (χ2 = 14.104, P = 0.001). Furthermore, the survival curves of 82 patients with mild and moderate APP are shown in Fig. 2. The Kaplan-Meier curve showed that no patients died within 8 days of admission, while from day 8 to day 28 of admission, the treatment of CHP could notably improve the survival rate of mild and moderate APP patients. Next, we used the Log Rank test to compare the Kaplan-Meier curve and found that the difference between curves was statistically significant (P = 0.000). The above experimental results showed that CHP treatment could improve the survival rate of patients better than HP treatment.

Survival curves of 82 patients with mild and moderate APP (Kaplan-Meier). The Kaplan-Meier curves of HP group (blue) and CHP group (green) within 28 days of admission (Log Rank P = 0.000).
Serum levels of PIIINP were measured in 82 patients with mild and moderate APP at the time of admission and the results are shown in Fig. 3. We observed that the patients whose blood PQ concentration was high also had a high serum level of PIIINP, suggesting there was a positive correlation between the level of PQ and PIIINP (r = 0.309, P = 0.000). PIIINP is released when type III procollagen is converted into type III collagen. It is elevated in the early stage of fibrosis and is an important indicator for evaluating early pulmonary fibrosis (Agarwal et al., 2014). The high level of PIIINP shown in Fig. 3 indicates that the higher blood concentration of PQ may induce more severe pulmonary fibrosis in mild and moderate APP patients.

The correlation between PIIINP level at admission and the concentration of PQ in patients with mild and moderate APP (n = 82).
To evaluate the therapeutic effect of CHP on pulmonary fibrosis caused by APP, we measured the level of PIIINP, CIV and TGF-β1 at pre-treatment, 3 and 7 days after treatment. As shown in Table 2, with the development of APP, the levels of PIIINP, CIV and TGF-β1 were increased gradually both in HP group and CHP group, while compared with HP group, the treatment of CHP could downregulate the levels of PIIINP, CIV, and TGF-β1 to some extent, suggesting that compared with HP, the CHP treatment could better relieve the fibrosis in lungs. Furthermore, the SOFA score also showed that the improvement effect on multiple organ failure of the CHP group was better than that of the HP group. To more intuitively observe the pathological changes of the lungs in APP patients, we performed HRCT on patients and selected several representative CT images. From the results of HRCT (Fig. 4), we observed ground-glass opacity (GGO) signal and interstitial changes in HRCT imaging, indicating that there was alveolar inflammation in the lungs at the early stage of APP both in the HP group and the CHP group. It’s worth noting that CHP treatment could reduce the inflammation in the lungs and at the 28 days after treatment, and that the GGO signal in the CHP group was less than that in the HP group, indicating the treatment of CHP had a better therapeutic effect on lung inflammation induced by APP. To further explore the mechanism of CHP relieving pulmonary fibrosis in patients with mild and moderate APP, we detected the content of MDA and the activity of SOD. As can be seen in Table 2, the MDA was increased and the SOD activity was reduced at 3 days after CHP treatment, while at 7 days after CHP treatment, this trend had been reversed, the decreased MDA and increased SOD activity hinting that CHP could inhibit oxidative stress response and thus slow down pulmonary fibrosis in mild and moderate APP patients.

| HP group (n = 40) | CHP group (n = 42) | ||||||
|---|---|---|---|---|---|---|---|
| PIIINP (ng/mL) | Pre-treatment | 104.57 | ± | 23.39 | 105.89 | ± | 23.51 |
| 3 days after treatment | 252.03 | ± | 53.09# | 225.56 | ± | 43.21*Δ | |
| 7 days after treatment | 348.23 | ± | 75.05& | 295.85 | ± | 68.61*▲ | |
| CIV (ng/mL) | Pre-treatment | 58.69 | ± | 11.78 | 59.52 | ± | 11.75 |
| 3 days after treatment | 205.61 | ± | 33.61# | 179.67 | ± | 30.22*Δ | |
| 7 days after treatment | 380.03 | ± | 43.92& | 344.12 | ± | 48.66*▲ | |
| TGF-β1 (pmol/L) | Pre-treatment | 109.59 | ± | 18.25 | 111.65 | ± | 18.19 |
| 3 days after treatment | 287.63 | ± | 31.61# | 246.22 | ± | 30.65*Δ | |
| 7 days after treatment | 495.41 | ± | 45.57& | 454.42 | ± | 43.52*▲ | |
| SOFA scores | Pre-treatment | 1.62 | ± | 0.22 | 1.64 | ± | 0.18 |
| 3 days after treatment | 7.69 | ± | 0.59# | 6.25 | ± | 0.64*Δ | |
| 7 days after treatment | 5.53 | ± | 0.52& | 4.47 | ± | 0.49*▲ | |
| MDA (nmol/mL) | Pre-treatment | 4.16 | ± | 0.35 | 4.17 | ± | 0.29 |
| 3 days after treatment | 9.31 | ± | 0.61# | 7.81 | ± | 0.49*Δ | |
| 7 days after treatment | 7.62 | ± | 0.73& | 6.36 | ± | 0.61*▲ | |
| SOD (U/mL) | Pre-treatment | 69.69 | ± | 8.25 | 69.06 | ± | 7.78 |
| 3 days after treatment | 37.82 | ± | 4.45# | 48.96 | ± | 6.26*Δ | |
| 7 days after treatment | 74.17 | ± | 12.34& | 86.37 | ± | 11.15▲ | |
#P < 0.05 vs pre-treatment of HP group, &P < 0.05 vs 3 days after treatment of HP group, ΔP < 0.05 vs pre-treatment of CHP group, ▲P < 0.05 vs 3 days after treatment of CHP group, * P < 0.05 vs HP group.

HRCT of APP patients in HP group and CHP group. a and b: 7 days after treatment of HP group; c: 28 days after treatment of HP group; d and e: 7 days after treatment of CHP group; f: 28 days after treatment of CHP group.
For APP patients, early diagnosis and treatment are the keys to improve their survival rate. In the present, we evaluated the therapeutic effect of HP and CHP on patients with mild and moderate APP, founding that early CHP treatment has a better effect in elevating the survival rate and alleviating pulmonary fibrosis of mild and moderate APP patients.
PQ is an organic heterocyclic herbicide with high toxicity to humans and animals. Oral poisoning is the main poisoning route. PQ can be distributed in the lungs, liver, kidneys, and other organs through blood circulation 2 hr after ingestion, causing multiple organ function damage and eventually death. Several researchers supported that plasma PQ concentration (within 2-4 hr after ingestion) could function as a marker of severity and prognosis (Senarathna et al., 2009; Cao et al., 2020). In line with the previous study, in our study, we also found that the blood PQ concentration at the admission of moderate APP patients was higher than that of mild APP patients, proving that blood PQ concentration was exactly a marker of the severity. For APP patients, early diagnosis and treatment are the keys to improve their survival rate. HP is an effective method to eliminate blood PQ, which is performed using activated charcoal and is effective for clearing protein-bound, lipid-soluble drugs and small water-soluble molecules. It has been reported that early HP treatment could markedly elevate survival rates and improve outcomes for PQ-poisoned patients (Wu et al., 2014). In this study, 24 hr after HP treatment, PQ still accumulated in the blood of mild and moderate APP patients, while CHP treatment could quickly remove PQ from the blood of patients within 8 hr, indicating that CHP could effectively eliminate blood PQ at the early stage of APP.
PQ contains a quaternary ammonium salt structure, which has similar functions to amines, and the alveolar epithelial cells contain an amine transport system, so PQ can be specifically absorbed by alveolar cells (Smith, 1982). Moreover, PQ can also enter pulmonary interstitial cells and pulmonary macrophages through the diffusion pathway (Dinis-Oliveira et al., 2008). Therefore, the lungs are the main target organ of APP, and the accumulation of PQ in the lungs can lead to acute lung injury and then development to pulmonary fibrosis gradually. TGF-β1 is considered to be an important cytokine in the formation process of pulmonary fibrosis, which can promote the fibroblasts differentiate into myofibroblasts, and promote the synthesis and deposition of collagen, thereby promoting the formation of fibrosis. Chen et al. (2018) reported that the inhibition of TGF-β1 could alleviate PQ induced pulmonary fibrosis by inhibiting epithelial-interstitial transformation. In our study, we found that compared with the HP group, the CHP treatment could downregulate the levels of TGF-β1 and CIV to some extent, suggesting that CHP treatment could better relieve the fibrosis in the lungs. In addition, oxidative stress is also an important cause of pulmonary fibrosis in APP patients. The accumulation of PQ in lung tissues induces the production of ROS, which further induces the peroxidation of surrounding lipids (Sun and Chen, 2016). Meanwhile, oxygen in alveoli can be converted into hydroxyl radicals through a series of catalysis, which can react with biological macromolecules and lead to DNA breakage, mitochondrial damage, activation of inflammatory cells, and ultimately induce the development of pulmonary fibrosis (Shen et al., 2017b). In our research, after CHP treatment, the level of MDA was reduced while the activity of SOD was elevated, suggesting CHP could reduce lipid peroxidation levels and eliminate oxygen free radicals. In conclusion, we believe that CHP treatment can relieve pulmonary fibrosis in APP patients by inhibiting the oxidative stress response.
In summary, APP patients emphasize early, comprehensive, regular, full-course treatment. CHP treatment can significantly reduce mortality and improve the prognosis of APP patients. On the one hand, early CHP treatment could enhance SOD activity and reduce MDA formation, thus inhibiting lipid peroxidation and reducing the production of oxygen free radicals. On the other hand, it can reduce PIIINP, CIV, TGF-β1, thus reducing the degree of pulmonary fibrosis. In addition, we found that the level of PIIINP in the blood of APP patients was positively correlated with PQ concentration. Because blood PIIINP level is one of the important indicators of pulmonary fibrosis, assessing the dynamic change of it in the process of APP treatment is helpful for monitoring change of condition, timely adjusting treatment options, and has important significance to guide the clinical medication. Taken together, our study clarified that compared with HP, CHP treatment exhibited a better therapeutic effect on mild and moderate APP patients, providing data support for the clinical promotion of CHP in the treatment of APP.
Conflict of interestThe authors declare that there is no conflict of interest.